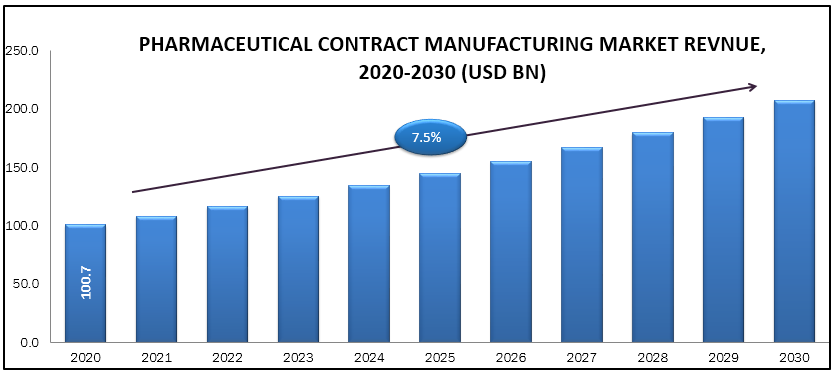
A contract manufacturing organization (CMO), helps various companies in the pharmaceutical industry on a contract basis to provide comprehensive services from drug development through drug manufacturing. It is also called as contract development and manufacturing organization (CDMO). This allows major pharmaceutical companies to outsource those aspects of the business, which can help them expanding or can allow the major company to focus on drug discovery and drug marketing. With the rising demand for generic biologics and medicines, the complex manufacturing requirements, the capital-intensive nature of the business, and many pharmaceutical companies have recognized the potential profitability in constricting with a CMO for both clinical and commercial stage manufacturing. The Pharmaceutical Contract Manufacturing market is accounted approximate USD 100.7 billion in 2020 and it is expected to reach approximate USD 207.5 billion by 2030 with a CAGR of 7.50 % during forecast period.
Market Segmentation:
The global pharmaceutical contract manufacturing market is segmented by service type into pharmaceutical, biologics, active pharma ingredients (APIs), tablet, capsule, parenteral, oral liquid, finished dosage formulation (FDF)). The market is classified by end-user into big pharma, small pharma, generic pharma, and CRO. By geography, the pharmaceutical contract manufacturing market is bifurcated into North America, Asia Pacific, Europe, and the RoW.
Market Dynamics and Factors:
Rising of pharmaceutical contract manufacturing market is driven by the growing of demand for generics, investments in advanced manufacturing technologies by CDMOs, and growing investments in pharmaceutical R&D. Most of the companies in this industry are progressively focusing on the development of biological APIs, which is boosting the market. General prescription drug sub-segment inhabits a major share in the API manufacturing segment, as compared to OTC drugs. However, stringent European regulatory policies are expected to hamper the segment’s growth. The novel technologies for HPAPIs (High Potent APIs) will potentially change the future market prospects for CMOs in this fast-growing segment.
Geographic Analysis:
Due to the growing manufacturing sector, increasing emphasis on off-patent drugs, favorable government regulations, and highly skilled workforce, all the factors are responsible for driving outsourcing of pharmaceutical contract development and manufacturing in the Asia Pacific and North America, which is the world’s largest market for drugs and accounts for almost half of the R&D spending in pharmaceutical and biotechnology markets. Therefore, CMOs play a major role in this market and have invested in new facilities and technologies to provide to a wide range of outsourcers. The U.S. is anticipated to face strong competition from Asia-Pacific CMO providers, especially in solid dose formation. Furthermore, the companies have been aggressive in expanding their services/ products/capabilities through strategic alliances. Acquisition of Confab, DPT Laboratories has become the global leader in prescription liquid and semi-solid formulations, obtaining proprietary products.
Competitive Scenario:
The major players in the pharmaceutical contract manufacturing market include Thermo Fisher Scientific Inc. (US), Catalent, Inc. (U.S.), Lonza Group Ltd (Switzerland), Recipharm AB (Sweden), Vetter Pharma International GMBH (Germany), FAMAR Health Care Services (Greece), AbbVie Inc. (US), Aenova Group (Germany), Consort Medical plc. (UK), Almac Group (UK), Siegfried Holding AG (Switzerland), Boehringer Ingelheim International GmbH (Germany), and Evonik Industries AG (Germany).
Pharmaceutical Contract Manufacturing Market Report Scope
| Report Attribute | Details |
| Analysis Period | 2020–2030 |
| Base Year | 2021 |
| Forecast Period | 2022–2030 |
| Market Size Estimation | Billion (USD) |
| Growth Rate (CAGR%) | 7.5 % |
|
| By Service (Pharmaceutical, Biologics, Active Pharma Ingredients (APIs), Tablet, Capsule, Parenteral, Oral Liquid, Finished Dosage Formulation (FDF)), By End User (Big Pharma, Small Pharma, Generic Pharma, and CRO) |
| Geographical Segmentation | North America (U.S., Canada, Mexico) Europe (UK, Germany, Italy, France, Rest of Europe), Asia-Pacific (China, Japan, India, Australia, Rest of APAC), South America (Brazil, Argentina, Rest of SA), MEA (UAE, Saudi Arabia, South Africa) |
| Key Companies Profiled | Thermo Fisher Scientific Inc. (US), Catalent, Inc. (U.S.), Lonza Group Ltd (Switzerland), Recipharm AB (Sweden), Vetter Pharma International GMBH (Germany), FAMAR Health Care Services (Greece), AbbVie Inc. (US), Aenova Group (Germany), Consort Medical plc. (UK), Almac Group (UK), Siegfried Holding AG (Switzerland), Boehringer Ingelheim International GmbH (Germany), and Evonik Industries AG (Germany). |